When does implementation research require ethics approval?
Authorship: The following blog was written by Shannon King, a MSPH candidate at Johns Hopkins Bloomberg School of Public Health who has been volunteering with SISN. The information reflects the current views of ethics in implementation put forward in the peer-reviewed literature.
Ensuring ethical standards are upheld is a core component of research within the scientific community. While ethical principles for clinical research have clearly been defined and regulated, implementation research (IR) presents its own ethical challenges that have yet to be fully comprehended and standardized. With the increasing burden on research ethics review boards, and the lengthy processing time for reviews (1,2), a clearer understanding of what data collection activities require review would help improve the efficiency of IRB committees and meet researchers’ needs to maintain ethical integrity.
As highlighted in SISN’s Integrated Framework for Implementation Science in Nutrition, IR encompasses a broad range of research objectives from initiation and scoping through to studies that examine commitment, support, financing and sustainability of interventions, and, in some cases, assessments of impact. For each study design, and goal, the ethical considerations may vary and might require different regulatory procedures. This report is based on ethical legislation in the United States for research involving human subjects; and will need to be modified in relation to other national regulations.
Defining Human Subject Research
As defined by the 45 Code of Federal Regulation part 46: Protection of Human Subjects, human subject research must meet both the definitions of “research” and “human subjects”. Research is “a systematic investigation including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.” Ultimately, any structured inquiry with a predetermined set of objectives is considered research and includes preliminary research, pilot studies for both intervention materials and intervention implementation, and intervention efficacy and efficiency studies. The second component of the definition is that it involves human subjects, defined as a “living individual about whom an investigator (whether professional or student) conducting research obtains 1) Data through intervention or interaction with the individual, or 2) Identifiable private information.” Identifiable information “includes information about behavior that occurs in a context in which an individual can reasonably expect that no observation is taking place, and information which has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public.”
Ethical issues to be considered when conducting Implementation Research
1. Type of study
An initial issue in ethical regulatory requirements for IR is the distinction between research and quality improvement (QI) activities (4, 5, 6). Programmatic quality improvements are seen as a less systematic approach and are not subjected to the same regulatory approvals from Institutional Review Boards (IRBs)*. However, some lines of quality improvement inquiry are considered research, for example those utilizing randomization. Thus, it is not always clear what distinguishes program research from quality improvement activities (5, 6). While some believe the difference between quality improvement and IR lie in the study characteristics, such as sample size, degree of documentation and type of information gathered (5); others classify QI as problem-driven, where they are motivated by quality gaps, whereas IR is solution-driven and motivated by an awareness that previously validated interventions are being under-utilized (6). Overall there is a lack of consensus about how to distinguish between research and quality improvements. This ambiguity creates uncertainty concerning what activities require IRB approval. As a result, several IRBs provide their own guidance on distinguishing between the two (7, 8). The following table highlights the main differences between QI and research:
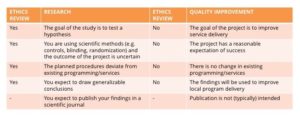 Adapted from Cahill N Preparing an Ethics Submission 2014
Adapted from Cahill N Preparing an Ethics Submission 2014
*Institutional Review Boards is the term for ethics approval committees in the USA
2. Nature of data being collected
The primary concern of ethics review boards is to ensure the protection of human subjects through minimizing the risks placed on the participants. Researchers need to clearly understand, define and explain the risks related to the data collection to obtain appropriate ethical review. Some researchers have found it helpful to examine whether the questions being asked are “about whom” or “about what” (5). “About whom” questions focus on the participants’ opinions, characteristics or behaviours and as such are considered higher risk and require ethics approval. However, “about what” questions, that involve non-personal aggregated information, for example asking about the overall outcomes or use of an intervention by the target population in general, pose less risk to participants, and as such may be exempt from requiring ethics approval. Furthermore, there are several review exemptions for specific categories of human subject research, such as educational testing, observations of public behaviours, and research involving previously collected data, when the data collected cannot be linked back to the research subject (13).
3. Publication of study results
It should be noted that the intention to publish the results of a study is not a requirement for IRB review. However, many journals require authors to include a comment on the ethical considerations of the study and if it received a full, expedited, or exempt review by the local IRB.
Steps to gaining ethics approval
While the flowchart below provides a useful guide to determining if a study is classified as human study research, many scenarios remain where this distinction is unclear. Consequently, the following steps represent best practice for upholding ethical standards:
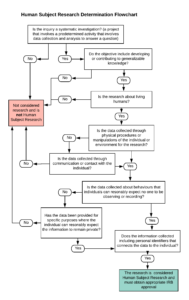
In order to maintain ethical integrity, all study protocols should uphold similar ethical standards regardless of if it is deemed to require a formal IRB review or not. Ethics approval should be obtained prior to commencing any stage of the research process and therefore it is important to initiate these steps early in the planning process (9).
Institutional Resources to Navigate Ethical Review Process
- Johns Hopkins School of Public Health. (2016). Guidance: determining public health practice from public health research
- Yale Interdisciplinary Center for Bioethics (n.d.). IBR case: public health practice vs. research
- Johns Hopkins Medicine. (2016). 102.1 Organization policy on determination of “human subject research” and exempt research
- Cornell University. (n.d). Institutional review board: frequently asked questions
- Harvard University (2017). Worksheet: human research determination
Have an idea or a comment on any of the issues discussed above? We welcome your feedback – you can comment on this post on our LinkedIn feed or write to us using the email address below.
To learn more about SISN and become a member, please contact us via our website (www.implementnutrition.org) or can email us at implementnutrition@gmail.com.

